Interfacial Step-Growth Polymerization
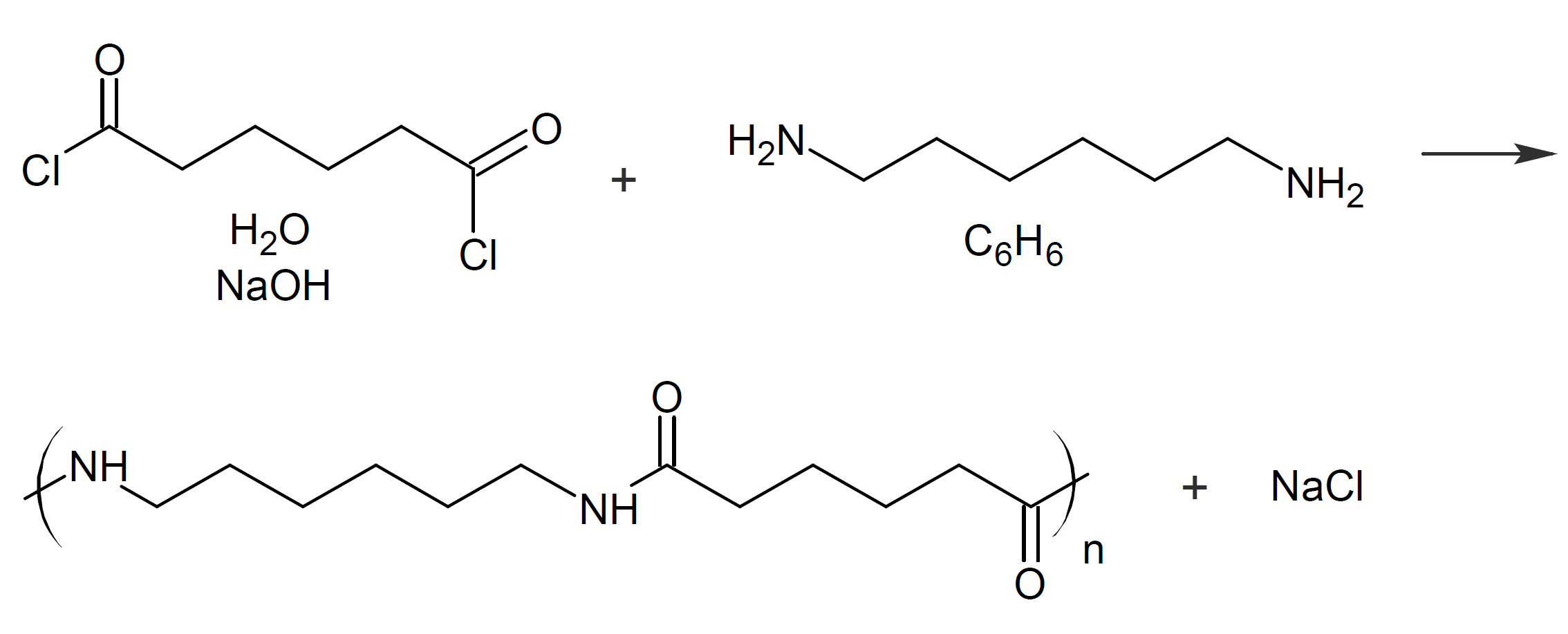
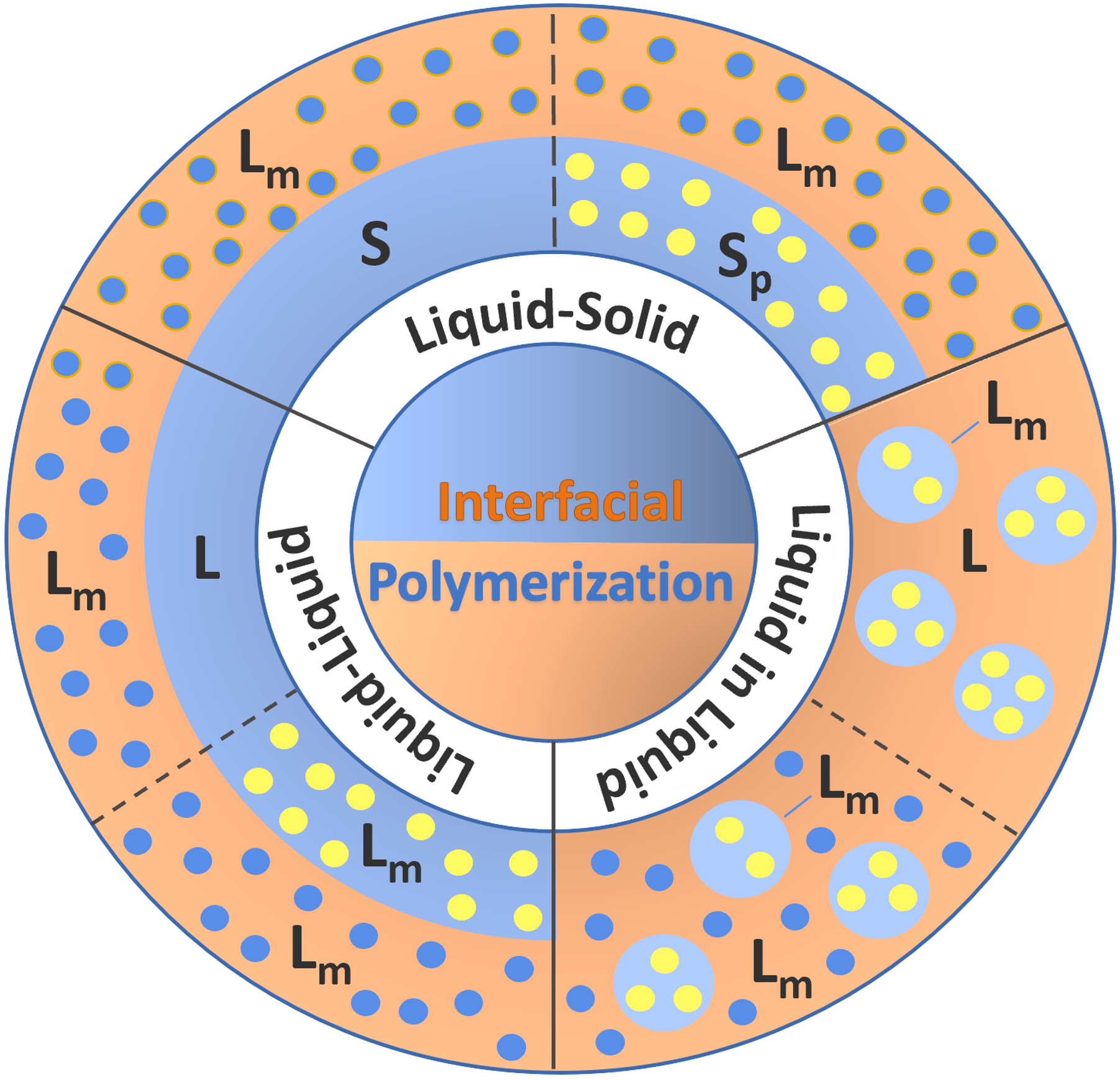
Interfacial polycondensation (IP) is a step growth polymerization of two or more monomers at the interface between two immiscible phases. This type of polymerization occurs when the monomers with different functionality are located in different phases. Often, the reaction occurs at a liquid-liquid interface. However, the polymerization can also occur at a liquid-gas or liquid-solid interphase. In the latter case, the solid could be a porous support (Sp) in which a monomer resides which is in contact with a dissolved liquid coreactant (Lm). In the case of polycondensation, liquid-liquid IP is the most common type. Often, one monomer or both monomers are dissolved in two immiscible liquids (Lm-L, Lm-Lm). A common example is the reaction of a diamine with a diacid chloride at the water/benzene interface.11 This type of IP has been extensively studied and was first described by Morgan, Kwolek and Beaman et al. (1959).1-3
To increase the surface area,
one of the phases may be dispersed in the other phase, which is
known as liquid-in-liquid interfacial polymerization.10 The continuous
phase can either be the co-reactant itself (L-in-Lm) or a solution
of it (Lm-in-Lm).
Sub-Classes of Interfaical Polymerization
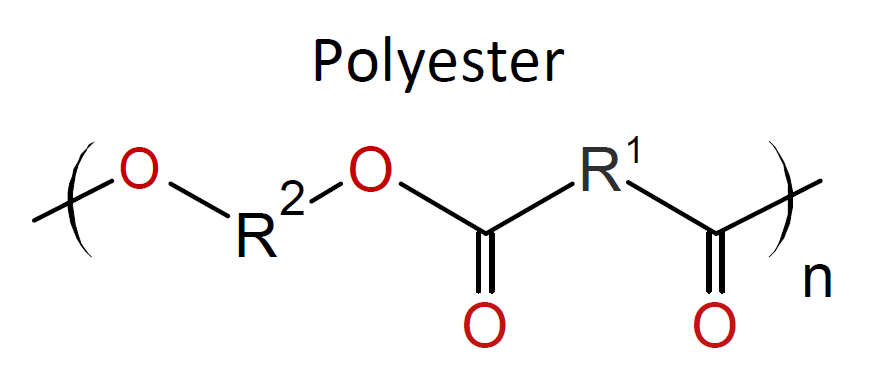
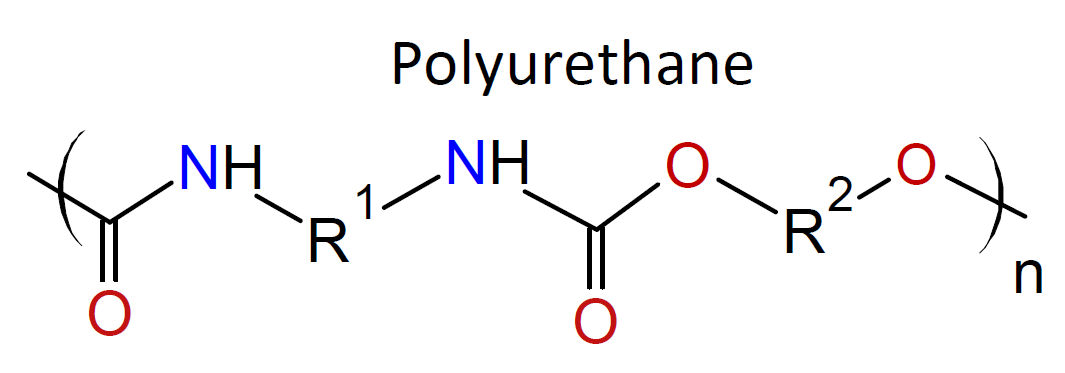
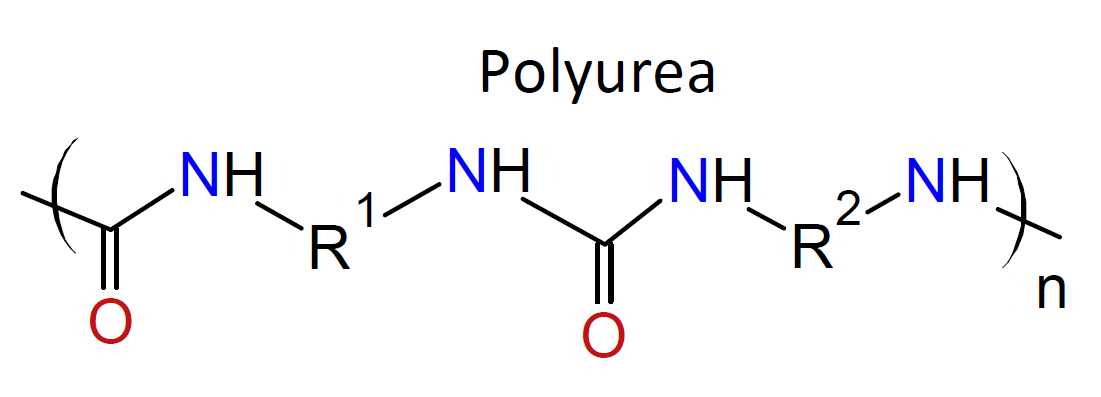
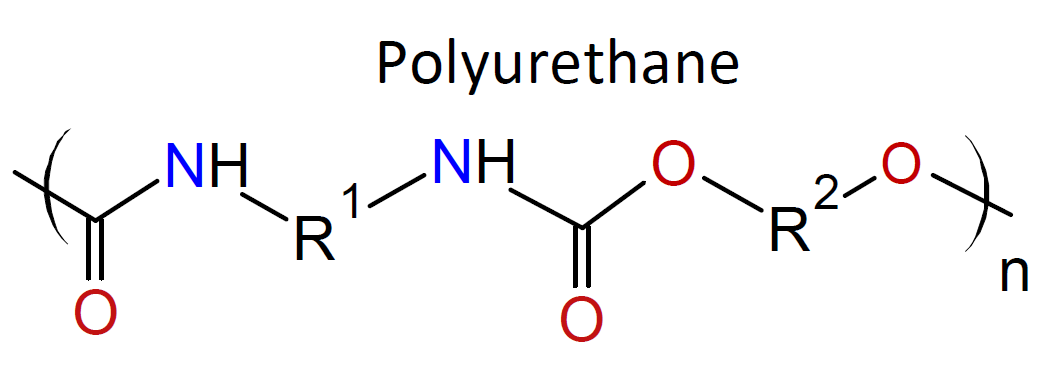
The IP method is suitable for a wide range of monomers and allows for the synthesis of a large variety of polymers including polyamides,
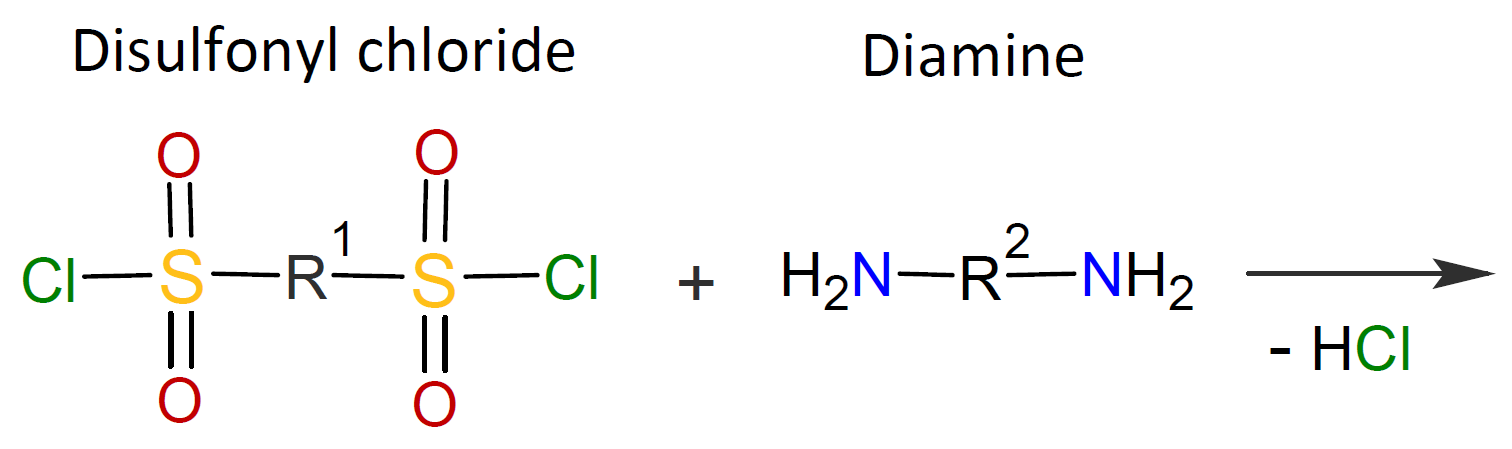
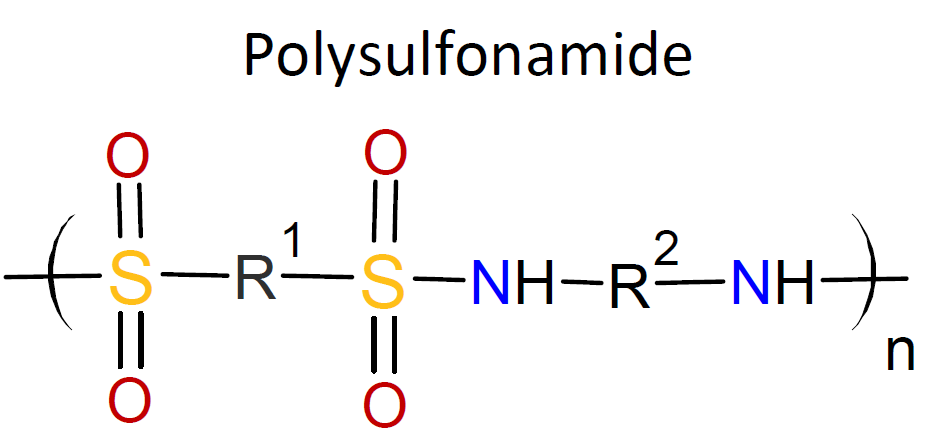
polyesters, polyurethanes, polyureas, polyimides, polysulfonamides and polycarbonates (see
examples in table below).4,5 It also allows for
microencapsulation of various materials and for the fabrication of a
variety of functional materials such as ultra thin metal-organic hybrid frameworks6,7
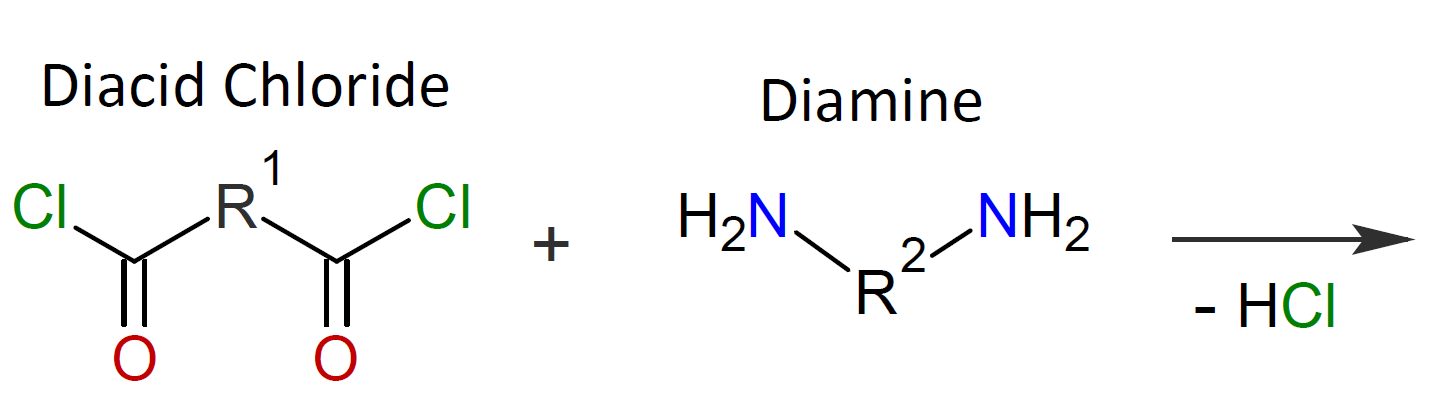
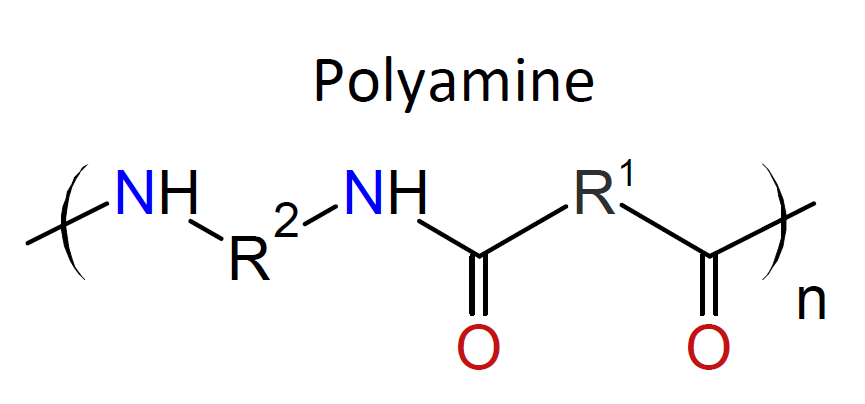
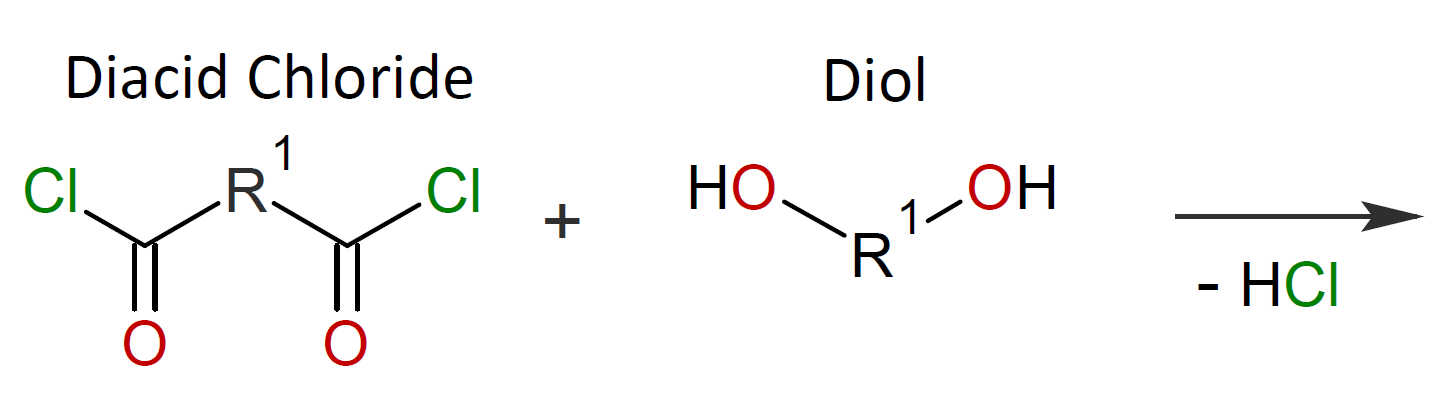
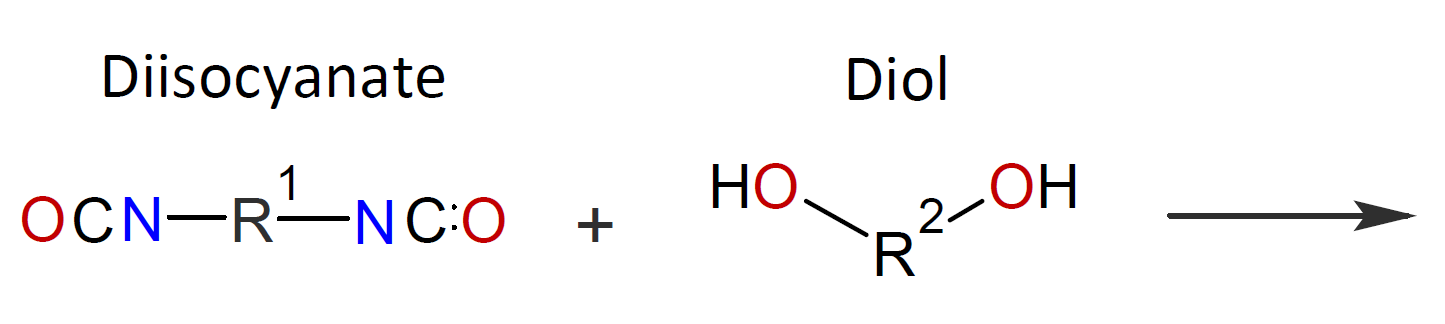
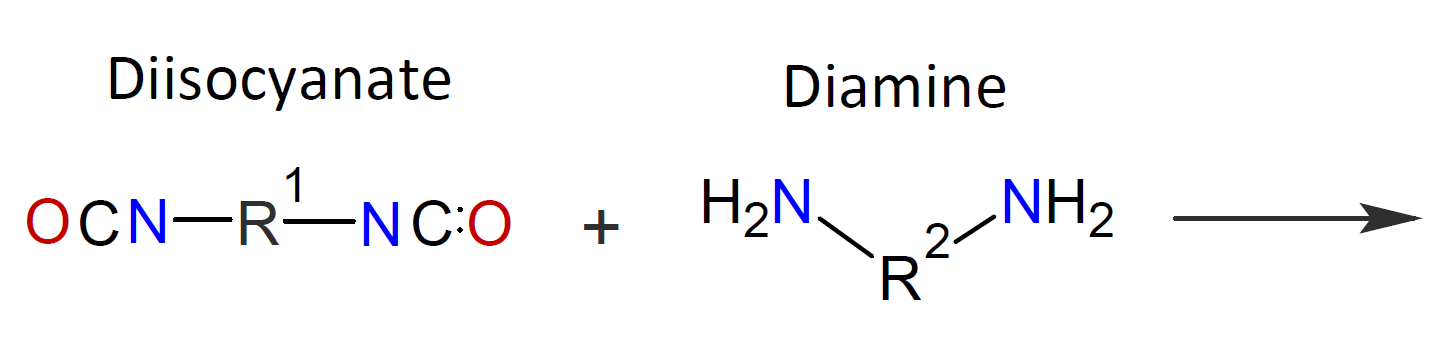
Typically, the monomers used in interfacial polymerization are of much higher reactivity than those used in common polycondensations.7 For example, in many IPs, di/tri acyl chlorides are employed which are much more reactive than the corresponding acids and esters used in conventional polycondensation. Some other monomers of high reactivity used in IPs include trichloride functionalized triazine, sulfuryl chloride, and diisocyanates which are most often reacted with diamines or diols (see table below).4-7
| Reaction | Product |
 |
 |
 |
 |
 |
 |
 |
 |
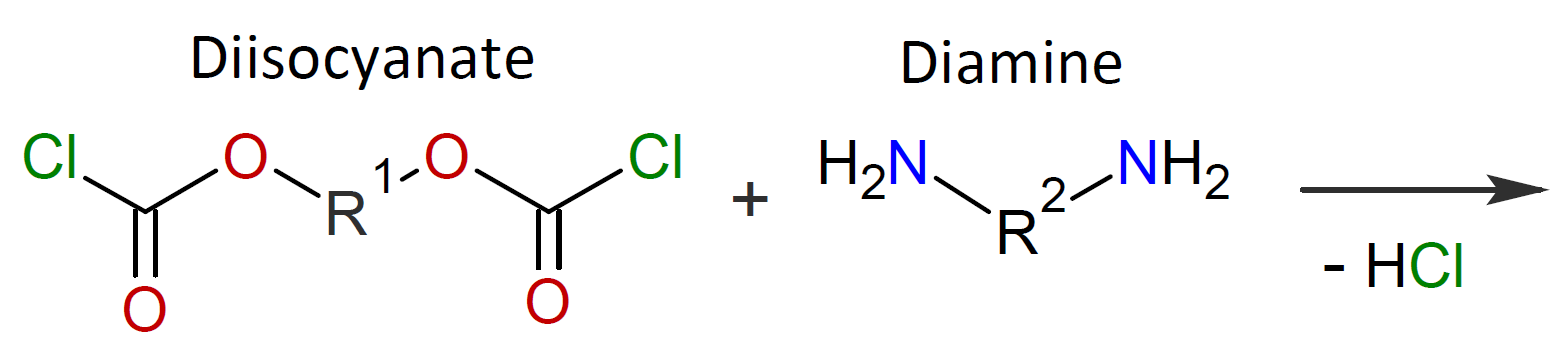 |
 |
 |
 |
Interfacial polymerization possesses several advantages over classical step-growth polymerization including high reaction rates at mild reaction conditions and high final molecular weights. Films fabricated by this method under controlled conditions are inherently uniform and free of defects.5 Furthermore, the morphology of the film can be controlled by adjusting the monomer concentration in the two adjoining phases, and by the solvent types.7
The final thickness of an IP film is controlled by the diffusion of monomers through the film (i.e.
by their permeability). Since the rate of diffusion steadily decreases with increasing film thickness, the process is self-limiting;
meaning, the film thickness grows rapidly in the beginning but then the growth rate steadily decreases and becomes zero at a certain film thickness
because the film itself forms a diffusion barrier for further interfacial polymerization.
Other factors that affect the rate of polymerization, the final
molecular weight, and the thickness and morphology of the formed film
include the solubility and concentration of the monomers,
their reactivity in the produced film as well as the solubility of the oligomers and polymers in the solvents.5 This makes the prediction
of the reaction rate and final film properties very difficult.
References & Notes
P.W. Morgan, and S.L. Kwolek, J. Polym. Sci., Vol. XL, 299-327 (1959)
R.G. Beaman, P.W. Morgan, C.R. Koller, E.L. Wittbecker, J. Polym. Sci., Vol. XL, 329-336 (1959)
P.W. Morgan and O.R. Strachan, US Patent 2,708,617 (1955)
C. Perignon, G. Ongmaye2, R. Neufeld, Y. Frere, D. Poncelet, J Microencapsul., 32 (1), 1-15 (2014)
E.I. Wittbecker and P.W. Morgan, J. Polym. Sci., Vol XL, 289-297 (1959)
N.E. Benes, M.J.T. Raaijmakers, Prog. Poly. Sci., 63, 86-142 (2016)
S. Wang, F. Zhang, and J.-B. Fan, Angew. Chem. Int., 10.1002/anie.201916473 (2020)
S. Behera and A.K. Suresh, Polymer, 127 28-44 (2017)
D.A Holmer, Thesis: A Study of the Mechanism of Interfacial Polyamidation and Polyesterification, Oklahoma State Univ. 1961
Y. Song, J.-B. Fan and Shutao Wang, Mater. Chem. Front, 1, 1028-1040 (2017)
The reaction of an amine with an acid chloride is generally known as the Schotten-Baumann reaction.
July 25, 2020